Please note: a valid prescription is required for all prescription medication.
Apretude® Injectable Suspension for HIV PrEP
$2,413.99
Secure Encrypted Payments
Apretude is a long-acting prescription injection used for HIV prevention in at-risk adults and adolescents. This page explains how it works, who it may suit, and how to plan clinic visits and refills. You can access Canadian pricing with US delivery from Canada, even if paying without insurance.
What Apretude Is and How It Works
Apretude® is a preventive HIV medicine containing cabotegravir. It belongs to the integrase inhibitor class and helps block the virus from entering human DNA. Cabotegravir injectable suspension is given in the gluteal muscle by a healthcare professional. It may be started with a short oral lead-in to check tolerability, or your prescriber may choose direct-to-inject based on your situation.
YouDrugstore is a licensed Canadian pharmacy in Manitoba. Pharmacists review prescriptions before dispensing.
This long-acting injectable is scheduled in the clinic, which can help with adherence. It works best when HIV testing remains negative and injections are kept on time. Discuss risk reduction strategies, including condoms, with your clinician.
Who It’s For
This medicine is for HIV pre-exposure prophylaxis in adults and adolescents at risk who weigh at least 35 kg. It should be used only after a documented negative HIV test before starting and prior to each injection. People with confirmed or suspected HIV infection should not use this preventive treatment.
Avoid use if you have had a previous severe allergic reaction to cabotegravir. Tell your prescriber about all medicines you take. Certain enzyme inducers can lower cabotegravir levels. Your clinician will help determine if this therapy is appropriate for you.
Dosage and Usage
A clinician gives each intramuscular injection in the buttock. The typical schedule includes one starting dose (month 0), a second dose one month later (month 1), then maintenance injections every two months.
Long-acting cabotegravir injection may be preceded by about four weeks of oral cabotegravir to assess tolerability. Your prescriber may also use a direct-to-injection start. Do not attempt self-injection. Keep all clinic appointments to maintain protection. If your schedule needs to change, contact your prescriber for guidance.
HIV testing is done before the first injection and before each follow-up dose. Tell your clinic right away if you have symptoms suggestive of acute HIV infection.
Strengths and Forms
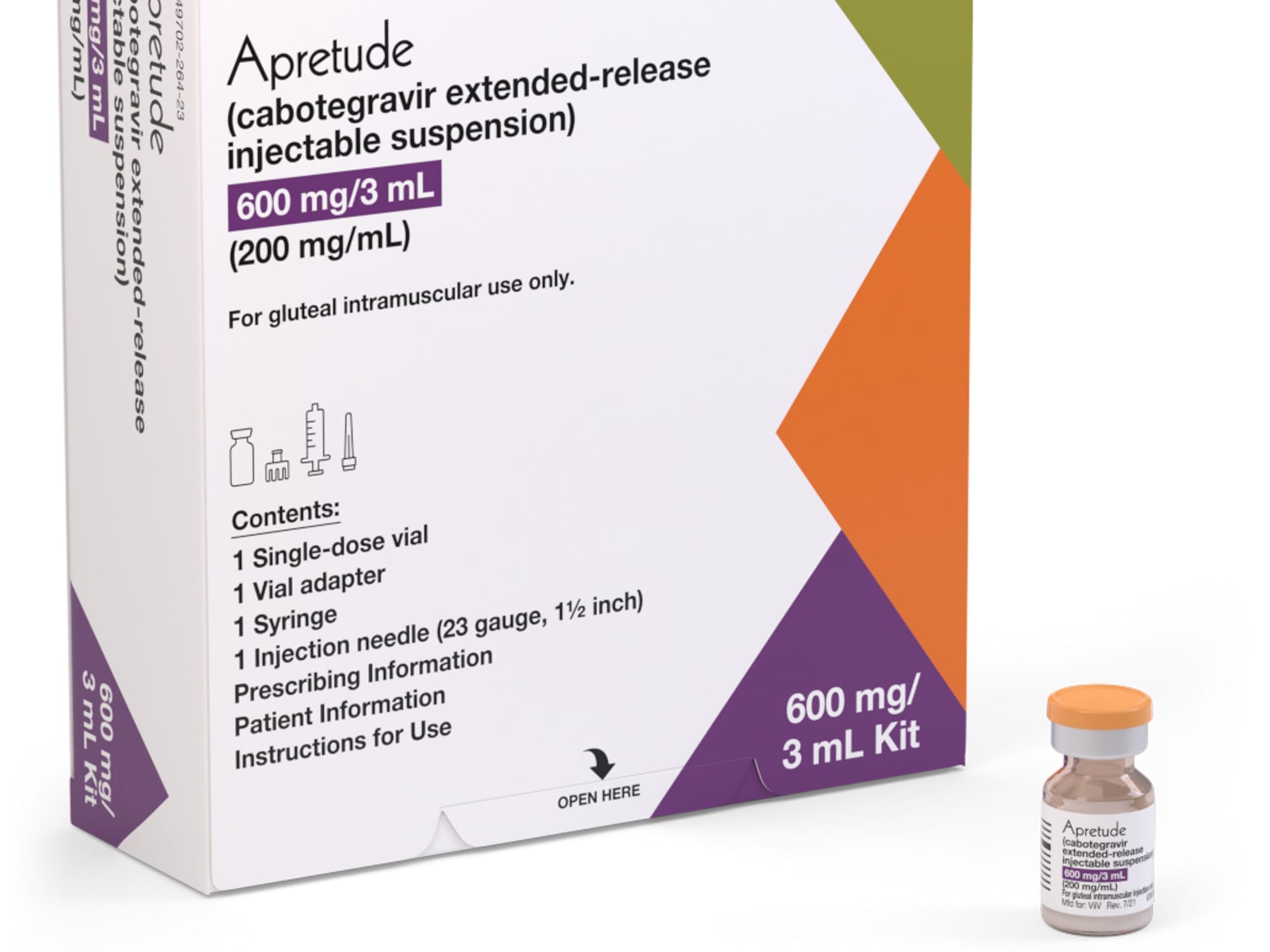
Apretude is supplied as a single-dose vial for intramuscular use. Availability can vary by package size and kit components. Check the current product listing for specific packaging details.
Apretude 600 mg injection is the commonly used dose in adults and adolescents who qualify for this therapy. Some sites may receive a complete kit for clinician use; others may stock individual vials. Your clinic team will manage preparation and administration.
Missed Dose and Timing
Try to receive each dose on the planned date. If you miss an appointment, contact your prescriber as soon as possible. The plan may include rescheduling your injection, using a short course of oral cabotegravir as a bridge, or restarting the series. The approach depends on how much time has passed. Do not self-administer or guess replacement timings.
Storage and Travel Basics
If you need to hold the vial before your appointment, keep it in the original carton, at room temperature as directed on the label, and out of reach of children. Do not freeze. Protect it from excessive heat and keep it upright until your clinic visit. Do not shake the vial.
For travel, bring your prescription, invoice, and clinic contact details. Keep the medicine in carry-on luggage in the original packaging. Allow extra time for security screening. If the trip overlaps with your injection window, arrange your clinic appointment in advance and ask about local options if needed. Your prescriber can advise on timing and any oral bridging if a trip causes a scheduling shift.
Benefits
This treatment can reduce reliance on daily pills and supports consistent clinic follow-up. Many people value scheduled appointments that reinforce adherence and routine HIV testing. The two-month maintenance interval after the second dose can fit well with busy routines. Clinic administration also confirms each dose was given, which some find reassuring.
Apretude PrEP injection may help people who prefer an infrequent dosing plan. It can be a practical option if remembering daily tablets is challenging. Your clinician will help decide if this approach fits your risk profile and health history.
Side Effects and Safety
- Injection site pain, swelling, or hardness
- Headache or fatigue
- Fever or feeling unwell
- Nausea or diarrhea
- Muscle or joint aches
- Sleep disturbance
- Rash
Serious reactions are uncommon but can include severe hypersensitivity, liver problems, depressive disorders, and significant injection site reactions. Because cabotegravir remains in the body for a long time, missed or delayed doses may allow the virus to develop resistance if HIV infection occurs. HIV testing before each dose is essential. Seek urgent care for signs of severe allergy, severe mood changes, or jaundice.
Drug Interactions and Cautions
Strong enzyme inducers can lower cabotegravir exposure and reduce effectiveness. Tell your prescriber if you use rifampin, rifapentine, carbamazepine, oxcarbazepine, phenytoin, or phenobarbital. They may choose a different strategy for prevention.
Use caution if you have liver disease or a history of depression. Discuss pregnancy plans and breastfeeding with your clinician. Let your team know if you take anticoagulants, as intramuscular injections can cause bleeding or bruising. Do not use this medicine if you have or may have acute HIV infection; a full treatment regimen is required in that situation.
What to Expect Over Time
After the initial two injections, most people transition to every-two-month visits. Injection site soreness is common and usually improves within a few days. Routine HIV testing continues before each injection, along with counseling on risk reduction. If you stop this therapy, cabotegravir can remain in the body for months. Your prescriber may recommend another preventive option during this period to reduce the chance of developing resistance if exposure occurs.
Compare With Alternatives
Oral PrEP is an option for many people who prefer tablets. Some choose a daily regimen such as Descovy® based on individual needs and eligibility. If HIV is diagnosed, care shifts to a complete treatment regimen, which may include options like Dovato® as determined by your prescriber. Your clinician can explain the differences, monitoring, and visit schedules for each path.
Pricing and Access
See current Canadian pricing and compare options for your prescription. Many patients ask about savings when paying cash. You can explore costs and potential savings for Apretude from Canada and coordinate clinic delivery. We offer Promotions that may help qualified orders. Ships from Canada to US. Encrypted checkout protects your information.
To review medicine options by condition or class, browse our categories for HIV and Antivirals. For background reading, see HIV Testing Day, Delstrigo Facts, and Genvoya Guide to learn more about prevention, testing, and treatment.
Availability and Substitutions
Supply can vary. If this item is unavailable, your prescriber may recommend a suitable alternative based on your history and risk profile. Do not switch or stop without guidance. Your clinician will also advise on continued HIV testing and any interim prevention strategy if a change is needed.
Patient Suitability and Cost-Saving Tips
Good candidates are those who prefer infrequent dosing, can attend clinic appointments, and have ongoing HIV-negative tests. This extended-release cabotegravir injection may be less suitable if you take strong enzyme inducers or cannot reliably return for scheduled visits.
Consider booking the next two appointments before you leave the clinic. Ask your prescriber about aligning appointments with travel. If allowed by your prescription, you may arrange multi-month planning so vials or a kit are ready ahead of time. Set reminders on your phone and keep a simple calendar of visits, lab testing, and renewal dates to avoid gaps.
Questions to Ask Your Clinician
- Is an oral lead-in right for me before the first injection?
- What signs of acute HIV infection should I watch for between visits?
- How should we handle a delayed or missed appointment?
- Do any of my medicines interact with this therapy?
- What lab tests do I need before each dose?
- How long should I continue prevention after my risk changes?
- What should I do if I plan extended travel around my injection window?
Authoritative Sources
Health Canada Drug Product Database
Ready to get started? Order with US shipping from Canada and prompt, express service with temperature-controlled handling when required. Talk to your prescriber if you have questions. This page is informational and does not replace medical advice or the approved label.
Express Shipping - from $25.00
Shipping with this method takes 3-5 days
Prices:
- Dry-Packed Products $25.00
- Cold-Packed Products $35.00
Shipping Countries:
- United States (all contiguous states**)
- Worldwide (excludes some countries***)
Standard Shipping - $15.00
Shipping with this method takes 5-10 days
Prices:
- Dry-Packed Products $15.00
- Not available for Cold-Packed products
Shipping Countries:
- United States (all contiguous states**)
- Worldwide (excludes some countries***)
Do I need an oral lead-in before the first injection?
Some people start with oral cabotegravir for about four weeks to check tolerability. Others may start directly with the injection when appropriate. Your prescriber will recommend the best approach based on your history and risk. Regardless of the path, a negative HIV test is required before beginning and before each dose. Do not start or stop any regimen without checking the official label and talking to your healthcare professional.
How often are the clinic visits for this preventive injection?
You will have an initial injection on day 0, a second dose one month later, and maintenance injections every two months. Visits include HIV testing and a brief assessment for side effects or interactions. Arrive on time and tell your clinician if you feel unwell or if any new medicines were started. If a visit must be delayed, contact your prescriber to discuss rescheduling or oral bridging support.
What are the most common side effects after the shot?
Injection site reactions are most common and can include pain, swelling, or hardness in the buttock. You might also notice headache, fatigue, fever, muscle aches, or nausea. These effects are usually mild to moderate and resolve within a few days. Serious reactions are rare but require medical attention, such as severe rash, jaundice, or mood changes. Report persistent or severe symptoms to your healthcare professional.
Which medicines can interfere with this HIV prevention therapy?
Strong enzyme inducers can reduce cabotegravir levels. Tell your prescriber if you use rifampin, rifapentine, carbamazepine, oxcarbazepine, phenobarbital, or phenytoin. Your clinician may recommend a different prevention plan if interactions are likely. Always bring an updated medication list to appointments, including supplements and over-the-counter products. Never stop or change any medicines without professional guidance.
What if I think I was exposed to HIV and missed my scheduled dose?
Contact your prescriber promptly. The plan may include testing and discussing oral bridging or rescheduling an injection, depending on timing. Because cabotegravir persists in the body, the clinician will consider the time since the last dose and your risk. Do not attempt self-injection or use another person’s medicine. Follow the official label and your clinician’s instructions for next steps.
Can adolescents receive this prevention shot?
Adolescents who weigh at least 35 kg and are at risk for HIV may be eligible, provided they have a negative HIV test before starting and before each dose. Suitability depends on medical history, ability to attend scheduled visits, and the absence of significant drug interactions. A parent or guardian may need to be involved depending on local regulations. The prescriber will confirm if this option is appropriate.
How should I store the vial if I’m transporting it to my clinic?
Keep the vial in its original carton at room temperature as directed on the label, out of reach of children. Do not freeze or expose it to extreme heat. Keep it upright and avoid shaking. When traveling, carry documentation such as your prescription and receipt, and keep the medicine in your hand luggage. If travel overlaps your dosing window, ask your prescriber about timing or temporary oral support.